SOFC
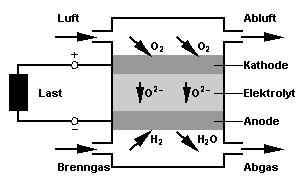
SOFC is the abbreviation for solid oxide fuel cell and designates a high temperature fuel cell with oxide ceramic electrolytes. The electrolyte in this type of fuel cell is a thin ion conducting ceramic plate with porous electron conducting electrodes on the top and bottom sides.
 |
 |
SOFC |
Principle of the SOFC |
The oxygen provided at the cathode side is reduced at the electrode to oxygen ions.
These ions travel through the ceramic plate, which conducts ions above about 800 C°,
to the anode where the oxidation of the fuel gas takes place.
The electrons generated by this process flow through the outer circuit back to the cathode.
The reaction products are dissipated with the fuel gas flow.
In order to increase the overall electric tension several cells are plugged together in a stack.
The functionality of oxide ceramic high temperature fuel cells critically depends on on the uniform supply
with fuel gas and air for each cell of the stack and the electrochemically active areas.
The mathematical model leads to a system of coupled nonlinear differential equations.
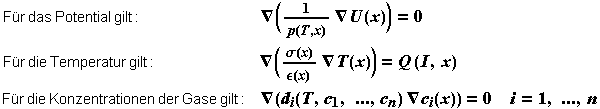 |
|
Some of the differential equations to be solved. |
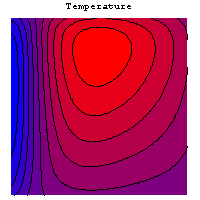
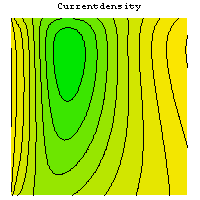
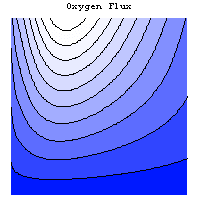
The simulation allows to investigate the dependence of the gas distribution on geometric parameters and its influence on various operating parameters. The following graphics show the temperature field, the current density distribution and the flux of oxygen in a single cell of a stack.
 |
 |
 |